Welcome to our first article on the pharmacology of metabolism. We will start with a medication known as Farxiga (dapagliflozin) of the SGLT2 inhibitor class. These drugs started life as “diabetes meds,” later showed strong evidence in heart and kidney disease, and now have emerging research suggesting weight loss and other benefits.
Who is this for: People with type 2 diabetes, heart failure (reduced or preserved EF), chronic kidney disease, or anyone trying to understand why one class of meds keeps showing up in cardio‑renal conversations.1
Important: this is education, not personal medical advice. Decisions about meds (especially with kidney function, diuretics, insulin, fasting/surgery, pregnancy, etc.) belong in a clinician‑patient conversation.1
Mechanism of Action
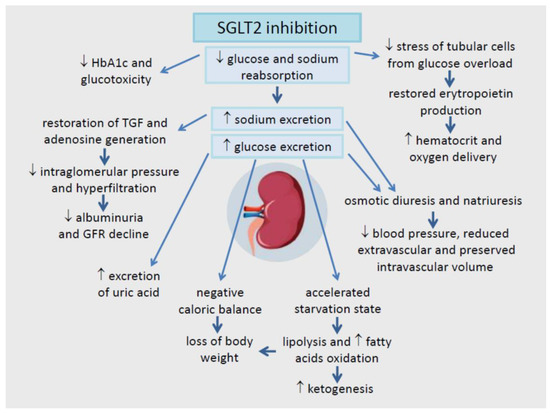
SGLT2 inhibitors block a glucose‑and‑sodium “recycling pump” (SGLT2) in the kidney’s proximal tubule, so more glucose (sugar) and some sodium exits via urine. That lowers blood sugar without relying on insulin, nudges blood pressure down, results in calorie loss (200–300 kcal/day) without reducing food intake, and translates into fewer heart failure events and slower kidney decline in the right patients.2–4,10,11
Original Purpose: Diabetes
For type 2 diabetes, dapagliflozin improves glycemic control as an adjunct to diet and exercise (and in combination with other agents). Because the mechanism is “dump glucose via urine,” it’s often described as insulin‑independent, making it less effected by pancreatic health and insulin resistance.1,9
What does that usually mean in practice? Across trials and reviews, SGLT2 inhibitors tend to lower HbA1c modestly (often roughly ~0.5–0.8% on average, depending on baseline and what they’re paired with) and modestly reduce weight and blood pressure, again on average.9–10
The kidney function reality check: As kidney function declines, the glucose‑lowering effect attenuates (less filtered glucose to “spill”), which is why the FDA label says Farxiga is not recommended for glycemic control when eGFR is below 45 mL/min/1.73m².1
Pediatrics note (US label): Farxiga is also indicated for glycemic control in pediatric patients aged 10 years and older with type 2 diabetes (with the usual caveats and clinician oversight).1
Heart Health Benefits (HFrEF and HFpEF)
Dapagliflozin demonstrated outcome benefits in heart failure with reduced ejection fraction (HFrEF) in the DAPA‑HF trial, compared with placebo, it reduced the risk of worsening heart failure or cardiovascular death, and these benefits were seen whether or not participants had diabetes.5
For heart failure with mildly reduced or preserved ejection fraction (HFmrEF/HFpEF), the DELIVER trial found dapagliflozin reduced the risk of worsening heart failure or cardiovascular death compared with placebo, again across diabetes status.6
Why is this good? It suggests the class is not merely “glucose medicine with side perks,” but a cardio‑renal physiology modulator—affecting volume handling, hemodynamics, and kidney‑heart interaction in ways that matter clinically.2–4
Kidney Health (Preservation):
Dapagliflozin improved kidney outcomes in chronic kidney disease (CKD) in DAPA‑CKD, reducing a composite outcome that included sustained eGFR decline, end‑stage kidney disease, or death from renal/cardiovascular causes (in a population that included people with and without diabetes).7
Mechanistically, a central (but not only) idea is tubuloglomerular feedback restoration: more sodium reaches the macula densa, which reduces maladaptive hyperfiltration and lowers intraglomerular pressure—protective over the long haul, even if there’s an early, small “hemodynamic dip” in eGFR in some people.3
Label scope note: The FDA label includes CKD and heart failure indications, and also lists specific CKD populations where Farxiga is not recommended (e.g., polycystic kidney disease) because expected benefit is limited / not established.1
Weight Loss:
When you excrete glucose, you excrete energy. Classic pharmacology discussions estimate urinary glucose loss can translate to roughly ~200–300 kcal/day in some contexts—especially early on and in hyperglycemia—so you’d expect steady weight loss forever.10
But humans are not closed systems. The body tends to compensate (often by increasing appetite/energy intake), so observed weight loss is typically modest rather than dramatic.10
Meta‑analytic estimates vary by population and drug, but a common ballpark for class‑associated weight reduction is on the order of several percent—useful, rarely life‑altering by itself, and best seen as part of a broader metabolic and weight loss strategy rather than a solo “weight loss drug.”20
Benefits, Ranked by Evidence Level
Tier 1 — “Hard outcomes / FDA‑label”
• Type 2 diabetes glycemic control (adults; also pediatric ≥10 years per label)1
• Heart failure (reduced or preserved EF): fewer worsening HF events (and related outcomes)1,5–6
• Chronic kidney disease: slower progression / fewer major renal outcomes in studied populations1,7
Tier 2 — “Strong, repeatable evidence signals”
Weight Loss. See above.
Modest blood pressure reduction. Ambulatory BP meta‑analysis data show average reductions on the order of ~3–4 mmHg systolic and ~1–2 mmHg diastolic over 24 hours—small individually, meaningful at scale, and often additive with other approaches.11
Lower uric acid and fewer gout flares (signal). Multiple analyses suggest SGLT2 inhibitors lower serum uric acid and are associated with reduced gout risk compared with some alternatives. It’s not why we use the class, but it’s a relevant side benefit for the right person.12–13
Lower risk of serious hyperkalemia (especially relevant in CKD/HF). Individual‑participant meta‑analysis of randomized trials has found SGLT2 inhibitors reduce serious hyperkalemia risk in higher‑risk populations—important because hyperkalemia is one of the reasons clinicians sometimes can’t use or can’t optimize other kidney/heart‑protective therapies.14
Hemoglobin/hematocrit increases (usually modest), with a safety reassurance. SGLT2 inhibitors can raise hemoglobin/hematocrit. A large cohort analysis found erythrocytosis was more prevalent after SGLT2i initiation vs certain comparators, but it was not associated with higher arterial or venous thrombosis risk in that dataset—reassuring, and still something a clinician monitors in context.15
Tier 3 — “Emerging / mixed”
Fatty liver / metabolic liver disease (signal). In people with type 2 diabetes and NAFLD, meta‑analysis of randomized trials suggests dapagliflozin may improve liver-related measures (e.g., enzymes) compared with controls—promising, not yet a primary labeled indication, and still evolving as the MASH field moves fast.16
Atrial fibrillation (AF) risk: evidence is mixed. Some meta‑analyses suggest a modest reduction in incident AF, while others find no statistically significant effect overall. For now, AF prevention is best considered “possible but not proven,” and not the core reason to choose an SGLT2 inhibitor.17–18
Obstructive sleep apnea (OSA): under investigation. There’s active research exploring whether SGLT2 inhibitors meaningfully improve OSA metrics (likely via weight/visceral fat, fluid shifts, and cardiometabolic changes), but this is not a settled clinical use case yet.19
Caveats
Most common annoyances: genital mycotic infections, urinary symptoms/UTIs, and volume depletion effects (thirst, lightheadedness), especially if you’re also on diuretics or are prone to dehydration.1
Serious but uncommon: ketoacidosis (including “euglycemic” DKA), rare necrotizing fasciitis of the perineum (Fournier gangrene), and acute kidney injury in susceptible settings—this is why clinicians give “sick day” rules and pause it during major illness/dehydration/fasting risk situations.1
Surgery / fasting note: The label advises withholding Farxiga for at least 3 days before major surgery or prolonged fasting, and restarting when clinically stable and eating again (your clinician guides this).1
Drug interactions (brief): hypoglycemia risk rises if combined with insulin or insulin secretagogues (dose adjustments may be needed); caution with diuretics (volume depletion); and the label advises monitoring lithium concentrations because SGLT2 inhibitors may lower lithium levels.1
Dosing
Typical dose used for cardio‑renal indications: 10 mg once daily (per label and the heart/kidney outcome trials).1
Kidney thresholds (very brief): Farxiga is not recommended for glycemic control if eGFR <45 mL/min/1.73m²; for CKD/HF indications, the label allows initiation down to eGFR 25 mL/min/1.73m² (with continuation guidance if kidney function declines further). This is exactly where individualized clinician judgment matters.1
Cost and Coverage
In the US, SGLT2 inhibitors are commonly covered—especially when there’s a labeled indication like type 2 diabetes, heart failure, or CKD—but plans differ, prior authorization is common, and copays can be all over the map. If cost is a barrier, ask your clinician’s office about coverage strategy and assistance options
Next: the pharmacology of GLP‑1s
Next up we’ll do GLP‑1 receptor agonists (and the newer dual incretins) with the same mechanistic lens—covering both obesity care and diabetes/cardiometabolic risk. SGLT2s and GLP‑1s often complement each other because they act on different bottlenecks (kidney glucose handling vs appetite/satiety and incretin signaling), and the outcome data in modern care keeps stacking.1–4
Work with us
If you’re in Oregon and want clinician‑guided, evidence‑based cardiometabolic care (including thoughtful use of newer medications when appropriate), you can book with Dawnbreaker Health here: dawnbreakerhealth.com#appointments. If you’re outside Oregon, reach out anyway (but do not book an appointment), state coverage and licensure can expand based on patient demand
References
1. FARXIGA (dapagliflozin) prescribing information. DailyMed (NIH/NLM). Updated December 22, 2025. Link
2. Vallon V. State-of-the-Art-Review: Mechanisms of Action of SGLT2 Inhibitors and Clinical Implications. Am J Hypertens. 2024. Full text (PMC)
3. Upadhyay A. SGLT2 Inhibitors and Kidney Protection: Mechanisms Beyond Tubuloglomerular Feedback. Kidney360. 2024;5(5):771–782. doi:10.34067/KID.0000000000000425. Full text (PMC)
4. Salvatore T, Galiero R, et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int J Mol Sci. 2022;23(7):3651. Full text (MDPI)
5. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA‑HF). N Engl J Med. 2019. NEJM
6. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction (DELIVER). N Engl J Med. 2022. NEJM
7. Heerspink HJL, Stefánsson BV, Correa‑Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease (DAPA‑CKD). N Engl J Med. 2020. NEJM
8. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes (DECLARE–TIMI 58). N Engl J Med. 2019. NEJM
9. Whaley JM, Tirmenstein M, et al. Targeting the kidney and glucose excretion with dapagliflozin. Diabetes Metab Syndr Obes. 2012. Full text (PMC)
10. Ferrannini G, et al. Energy Balance After Sodium–Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015. Full text (PMC)
11. Georgianos PI, Agarwal R. Ambulatory Blood Pressure Reduction With SGLT‑2 Inhibitors: Dose‑Response Meta‑analysis. Diabetes Care. 2019. Full text (PMC)
12. Banerjee M, et al. Serum uric acid lowering and effects of SGLT2 inhibitors on gout: systematic review/meta‑analysis. 2023. PubMed
13. Zhang L, et al. Effects of SGLT‑2 inhibitors on serum uric acid levels in CKD: systematic review/network meta‑analysis. 2024. PubMed
14. Neuen BL, Oshima M, Agarwal R, et al. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Hyperkalemia in People With Type 2 Diabetes: A Meta-Analysis of Individual Participant Data From Randomized, Controlled Trials. Circulation. 2022;145(19):1460–1470. doi:10.1161/CIRCULATIONAHA.121.057736. PubMed
15. Lewis M, et al. SGLT2 Inhibitors, Erythrocytosis, and Thrombosis in Adults With Type 2 Diabetes. JAMA Netw Open. 2025. Full text (PMC)
16. Duan H, et al. Efficacy of dapagliflozin to treat NAFLD in patients with T2D: meta‑analysis. 2025. Full text (PMC)
17. Mariani MV, et al. SGLT2i effect on atrial fibrillation: network meta‑analysis. 2024. PubMed
18. Zhang HD, et al. SGLT2 inhibitors for prevention of atrial fibrillation: meta‑analysis. 2024. PubMed
19. Xie L, et al. DAHOS study protocol: dapagliflozin in HFrEF with obstructive sleep apnea. Trials. 2023. Full text (PMC)
20. Cheong AJY, et al. SGLT inhibitors on weight and body mass: A meta-analysis of 116 randomized-controlled trials. Obesity (Silver Spring). 2022;30(1):117–128. doi:10.1002/oby.23331. PubMed

No responses yet